Calcium carbonate is one of the most popular chemicals which is first encountered in school classrooms, where the use of chalk (a form of CaCO3) is found. It is found in the earth’s crust. It is also found in many forms such as marble, limestone, etc. Although they are available in various forms they are chemically similar and only differ physically. They are also referred to as calcite.
Commercial Production of Calcium Carbonate
Calcium carbonate is produced commercially in two different grades. Both grades compete industrially based primarily on particle size and the characteristics imparted to a product.
- Ground Calcium Carbonate – Produced via extraction and processing of naturally occurring deposits. GCC crystal shape is irregularly rhombohedral and has a broader size distribution.
- Precipitated Calcium Carbonate – Produced via chemical precipitation via a carbocation process or as a by-product of some bulk chemical processes. PCC crystal shape depends on the product and the particles are more uniform and regular with a narrow size distribution.
PCC has smaller particles has a higher purity is less abrasive and tends to have higher brightness than GCC.
Calcium Carbonate Structure
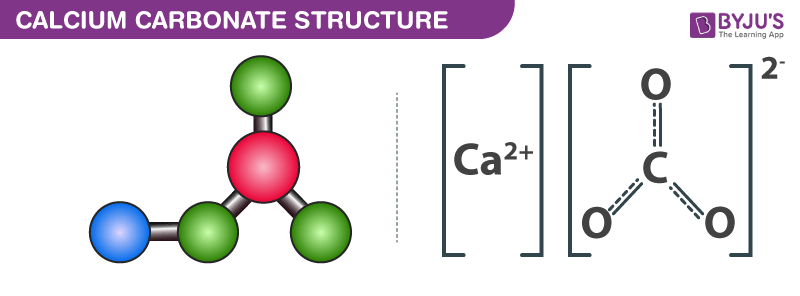
Structure of Calcium Carbonate
Calcium Carbonate Formula
- It is a chemical compound with the chemical formula CaCO3.
- It is a white insoluble powder-like substance which occurs naturally in minerals, chalk, marble, limestone, calcite, shells, pearl, etc.
- Medicinally, it is used as an antacid or as a calcium supplement. It is also used as filler in cosmetics. It is added to swimming pools as a disinfectant agent and a pH corrector.
- It finds extensive usage in the manufacturing industry as a building material (marble), ingredient for quick lime and cement.